 Hi Everybody!!
I am sharing this campfire to warm up all my cold friends! It was very cold this day when I burned the big tree that had to be cut down. I was glad to be close to the warmth for awhile. As you know, when you gaze into a fire, your mind tends to wander. I was thinking about how long people have been cooking with fire. (Of course, I looked it up in Google Search). Turns out people have been cooking with fire longer than humans have been on Earth! Like hundreds of thousands of years longer. So who was cooking? The monkeys? No other known species has ever used fire. That's why I have decided to let somebody else figure out this 'fire news' and tell me later. I have shared an excerpt from Wikipedia on Fire. Maybe it will light a spark on some of You to find out a little more for yourself!
Your photostudy is from this afternoon, when finally the sun broke through and the buzzards got busy drying their feathers. Enjoy!
Hi Everybody!!
I am sharing this campfire to warm up all my cold friends! It was very cold this day when I burned the big tree that had to be cut down. I was glad to be close to the warmth for awhile. As you know, when you gaze into a fire, your mind tends to wander. I was thinking about how long people have been cooking with fire. (Of course, I looked it up in Google Search). Turns out people have been cooking with fire longer than humans have been on Earth! Like hundreds of thousands of years longer. So who was cooking? The monkeys? No other known species has ever used fire. That's why I have decided to let somebody else figure out this 'fire news' and tell me later. I have shared an excerpt from Wikipedia on Fire. Maybe it will light a spark on some of You to find out a little more for yourself!
Your photostudy is from this afternoon, when finally the sun broke through and the buzzards got busy drying their feathers. Enjoy!

link to photostudy in G+Album:
https://plus.google.com/u/0/photos/117645114459863049265/albums/5976759109581107425
 https://en.wikipedia.org/wiki/Fire
https://en.wikipedia.org/wiki/Fire
Fire
From Wikipedia, the free encyclopedia
The flame is the visible portion of the fire. If hot enough, the gases may become ionized to produce plasma.[2] Depending on the substances alight, and any impurities outside, thecolor of the flame and the fire's intensity will be different.
Fire in its most common form can result in conflagration, which has the potential to cause physical damage through burning. Fire is an important process that affects ecological systems across the globe. The positive effects of fire include stimulating growth and maintaining various ecological systems. Fire has been used by humans for cooking, generating heat, signaling, and propulsion purposes. The negative effects of fire include water contamination, soil erosion, atmospheric pollution and hazard to life and property.[3]

Physical properties
Chemistry
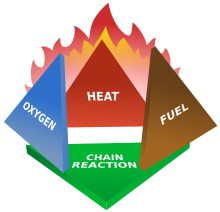
Fires start when a flammable and/or a combustible material, in combination with a sufficient quantity of an oxidizer such as oxygen gas or another oxygen-rich compound (though non-oxygen oxidizers exist that can replace oxygen), is exposed to a source ofheat or ambient temperature above the flash point for the fuel/oxidizer mix, and is able to sustain a rate of rapid oxidation that produces a chain reaction. This is commonly called thefire tetrahedron. Fire cannot exist without all of these elements in place and in the right proportions. For example, a flammable liquid will start burning only if the fuel and oxygen are in the right proportions. Some fuel-oxygen mixes may require a catalyst, a substance that is not directly involved in any chemical reaction during combustion, but which enables the reactants to combust more readily.
Once ignited, a chain reaction must take place whereby fires can sustain their own heat by the further release of heat energy in the process of combustion and may propagate, provided there is a continuous supply of an oxidizer and fuel.
If the oxidizer is oxygen from the surrounding air, the presence of a force of gravity, or of some similar force caused by acceleration, is necessary to produce convection, which removes combustion products and brings a supply of oxygen to the fire. Without gravity, a fire rapidly surrounds itself with its own combustion products and non-oxidizing gases from the air, which exclude oxygen andextinguish it. Because of this, the risk of fire in a spacecraft is small when it is coasting in inertial flight. Of course, this does not apply if oxygen is supplied to the fire by some process other than thermal convection.
Fire can be extinguished by removing any one of the elements of the fire tetrahedron. Consider a natural gas flame, such as from a stovetop burner. The fire can be extinguished by any of the following:
- turning off the gas supply, which removes the fuel source;
- covering the flame completely, which smothers the flame as the combustion both uses the available oxidizer (the oxygen in the air) and displaces it from the area around the flame with CO2;
- application of water, which removes heat from the fire faster than the fire can produce it (similarly, blowing hard on a flame will displace the heat of the currently burning gas from its fuel source, to the same end), or
- application of a retardant chemical such as Halon to the flame, which retards the chemical reaction itself until the rate of combustion is too slow to maintain the chain reaction.
In contrast, fire is intensified by increasing the overall rate of combustion. Methods to do this include balancing the input of fuel and oxidizer to stoichiometric proportions, increasing fuel and oxidizer input in this balanced mix, increasing the ambient temperature so the fire's own heat is better able to sustain combustion, or providing a catalyst; a non-reactant medium in which the fuel and oxidizer can more readily react.
Flame

Photo of a fire taken with a 1/4000th of a second exposure
A flame is a mixture of reacting gases and solids emitting visible, infrared, and sometimesultraviolet light, the frequency spectrum of which depends on the chemical composition of the burning material and intermediate reaction products. In many cases, such as the burning of organic matter, for example wood, or the incomplete combustion of gas, incandescent solid particles calledsoot produce the familiar red-orange glow of 'fire'. This light has a continuous spectrum. Complete combustion of gas has a dim blue color due to the emission of single-wavelength radiation from various electron transitions in the excited molecules formed in the flame. Usually oxygen is involved, but hydrogen burning in chlorine also produces a flame, producing hydrogen chloride(HCl). Other possible combinations producing flames, amongst many, are fluorine and hydrogen, and hydrazine and nitrogen tetroxide.
The glow of a flame is complex. Black-body radiation is emitted from soot, gas, and fuel particles, though the soot particles are too small to behave like perfect blackbodies. There is also photonemission by de-excited atoms and molecules in the gases. Much of the radiation is emitted in the visible and infrared bands. The color depends on temperature for the black-body radiation, and on chemical makeup for the emission spectra. The dominant color in a flame changes with temperature. The photo of the forest fire in Canada is an excellent example of this variation. Near the ground, where most burning is occurring, the fire is white, the hottest color possible for organic material in general, or yellow. Above the yellow region, the color changes to orange, which is cooler, then red, which is cooler still. Above the red region, combustion no longer occurs, and the uncombusted carbon particles are visible as black smoke.
The common distribution of a flame under normal gravity conditions depends on convection, as soot tends to rise to the top of a general flame, as in a candle in normal gravity conditions, making it yellow. In micro gravity or zero gravity,[4] such as an environment in outer space, convection no longer occurs, and the flame becomes spherical, with a tendency to become more blue and more efficient (although it may go out if not moved steadily, as the CO2 from combustion does not disperse as readily in micro gravity, and tends to smother the flame). There are several possible explanations for this difference, of which the most likely is that the temperature is sufficiently evenly distributed that soot is not formed and complete combustion occurs.[5] Experiments byNASA reveal that diffusion flames in micro gravity allow more soot to be completely oxidized after they are produced than diffusion flames on Earth, because of a series of mechanisms that behave differently in micro gravity when compared to normal gravity conditions.[6] These discoveries have potential applications in applied science and industry, especially concerning fuel efficiency.
In combustion engines, various steps are taken to eliminate a flame. The method depends mainly on whether the fuel is oil, wood, or a high-energy fuel such as jet fuel.
Heat
Fires give off heat, or the process of energy transfer from one body or system due to thermal contact.
Typical temperatures of fires and flames
- Oxyhydrogen flame: 2000 °C or above (3600 °F)[7]
- Bunsen burner flame: 1,300 to 1,600 °C (2,400 to 2,900 °F)[8]
- Blowtorch flame: 1,300 °C (2,400 °F)[9]
- Candle flame: 1,000 °C (1,800 °F)
- Smoldering cigarette:
- Temperature without drawing: side of the lit portion; 400 °C (750 °F); middle of the lit portion: 585 °C (1,100 °F)
- Temperature during drawing: middle of the lit portion: 700 °C (1,300 °F)
- Always hotter in the middle.
Temperatures of flames by appearance
The temperature of flames with carbon particles emitting light can be assessed by their color:[10]
- Red
- Just visible: 525 °C (980 °F)
- Dull: 700 °C (1,300 °F)
- Cherry, dull: 800 °C (1,500 °F)
- Cherry, full: 900 °C (1,700 °F)
- Cherry, clear: 1,000 °C (1,800 °F)
- Orange
- Deep: 1,100 °C (2,000 °F)
- Clear: 1,200 °C (2,200 °F)
- White
- Whitish: 1,300 °C (2,400 °F)
- Bright: 1,400 °C (2,600 °F)
- Dazzling: 1,500 °C (2,700 °F)
Fire ecology
Main article: Fire ecology
Every natural ecosystem has its own fire regime, and the organisms in those ecosystems are adapted to or dependent upon that fire regime. Fire creates a mosaic of different habitat patches, each at a different stage of succession.[11] Different species of plants, animals, and microbes specialize in exploiting a particular stage, and by creating these different types of patches, fire allows a greater number of species to exist within a landscape.
For other News on Fire, check Google Search.
Example:
Fox News - 5 days ago
A team of researchers has uncovered the oldest hearth in Israel, a300,000-year-old fire pit where prehistoric humans roasted ancient meats.










Link to photostudy in G+Albums:
https://plus.google.com/u/0/photos/117645114459863049265/albums/5976762039033896577









Link to photostudy in G+Album:
https://plus.google.com/u/0/photos/117645114459863049265/albums/5976766595714119377

 ...this is brendasue signing off from Rainbow Creek. See You next time!
...this is brendasue signing off from Rainbow Creek. See You next time!

O+O





























Very useful post. This is my first time i visit here. I found so many interesting stuff in your blog especially its discussion. Really its great article. Keep it up.
ReplyDeletelearn more