Hi Everybody!!
Well, well, well, well, well, well, well: Even MORE discovered about the Smart Clouds I have been photographing. Not only was the patent for this work applied for in 1946 (before I was born),
but also the development of this program has been going on for along time. They have been spraying the Instant Cloud Dust since 2009 and making clouds (see below). Now Guess who has been doing this????? Hint: Begins with N and ends with A!!!!!!
http://www.youtube.com/watch?v=PNBnfqUycto
Rayleigh scattering
From Wikipedia, the free encyclopedia
Rayleigh scattering, named after the British physicist Lord Rayleigh,[1] is the elastic scattering of light or otherelectromagnetic radiation by particles much smaller than the wavelength of the light. The particles may be individual atoms or molecules. It can occur when light travels through transparent solids and liquids, but is most prominently seen in gases. Rayleigh scattering results from the electric polarizability of the particles. The oscillating electric field of a light wave acts on the charges within a particle, causing them to move at the same frequency. The particle therefore becomes a small radiating dipole whose radiation we see as scattered light.
Rayleigh scattering of sunlight in the atmosphere causes diffuse sky radiation, which is the reason for the blue color of the sky and the yellow tone of the sun itself.
Scattering by particles similar to or larger than the wavelength of light is typically treated by the Mie theory, thediscrete dipole approximation and other computational techniques. Rayleigh scattering applies to particles that are small with respect to wavelengths of light, and that are optically "soft" (i.e. with a refractive index close to 1). On the other hand, Anomalous Diffraction Theory applies to optically soft but larger particles.
Small size parameter approximation
The size of a scattering particle is parameterized by the ratio x of its characteristic dimension r and wavelength λ:
Rayleigh scattering can be defined as scattering in the small size parameter regime x ≪ 1. Scattering from larger spherical particles is explained by the Mie theory for an arbitrary size parameter x. For small x the Mie theory reduces to the Rayleigh approximation.
The amount of Rayleigh scattering that occurs for a beam of light depends upon the size of the particles and the wavelength of the light. Specifically, the intensity of the scattered light varies as the sixth power of the particle size, and varies inversely with the fourth power of the wavelength.[2]
The intensity I of light scattered by a single small particle from a beam of unpolarized light of wavelength λ and intensity I0 is given by:
where R is the distance to the particle, θ is the scattering angle, n is the refractive index of the particle, and d is the diameter of the particle. The Rayleigh scattering cross-section is given by
The Rayleigh scattering coefficient for a group of scattering particles is the number of particles per unit volume N times the cross-section. As with all waveeffects, for incoherent scattering the scattered powers add arithmetically, while for coherent scattering, such as if the particles are very near each other, the fields add arithmetically and the sum must be squared to obtain the total scattered power.
[edit]From molecules
Rayleigh scattering also occurs from individual molecules. Here the scattering is due to the molecularpolarizability α, which describes how much the electrical charges on the molecule will move in an electric field. In this case, the Rayleigh scattering intensity for a single particle is given by[4]
The amount of Rayleigh scattering from a single particle can also be expressed as a cross section σ. For example, the major constituent of the atmosphere, nitrogen, has a Rayleigh cross section of 5.1×10−31 m2at a wavelength of 532 nm (green light).[5] This means that at atmospheric pressure, about a fraction 10−5 of light will be scattered for every meter of travel.
The strong wavelength dependence of the scattering (~λ−4) means that shorter (blue) wavelengths are scattered more strongly than longer (red) wavelengths. This results in the indirect blue light coming from all regions of the sky. Rayleigh scattering is a good approximation of the manner in which light scattering occurs within various media for which scattering particles have a small size parameter.
[edit]Reason for the blue color of the sky
Further information: Diffuse sky radiation
A portion of the beam of light coming from the sun scatters off molecules of gas and other small particles in the atmosphere. It is this scattered light that gives the surrounding sky its brightness and its color. As previously explained, Rayleigh scattering is inversely proportional to the fourth power of wavelength, so that shorter wavelength violet and blue light will scatter more than the longer wavelengths (yellow and especially red light). The resulting color, which appears like a pale blue, actually is a mixture of all the scattered colors, mainly blue and green. Conversely, glancing toward the sun, the colors that were not scattered away — the longer wavelengths such as red and yellow light — are directly visible, giving the sun itself a slightly yellowish hue. Viewed from space, however, the sky is black and the sun is white.
The reddening of sunlight is intensified when the sun is near the horizon, because the volume of air through which sunlight must pass is significantly greater than when the sun is high in the sky. The Rayleigh scattering effect is thus increased, removing virtually all blue light from the direct path to the observer. The remaining unscattered light is mostly of a longer wavelength, and therefore appears to be orange.
Rayleigh scattering primarily occurs through light's interaction with air molecules. Or, from a purely macroscopic point of view, blue sky comes from microscopic density fluctuations, resulting from the random motion of molecules in the air. A region of higher or lower density has a slightly different refractive index from the surrounding medium, and therefore it acts like a short-lived particle that scatters light in random directions. Smaller regions fluctuate more than larger ones, and, since short wavelengths are disturbed by small regions more than longer wavelengths, they are scattered more.
Some of the scattering can also be from sulfate particles. For years after large Plinian eruptions, the blue cast of the sky is notably brightened by the persistent sulfate load of the stratospheric gases. Some works of the artist J. M. W. Turner may owe their vivid red colours to the eruption of Mount Tamborain his lifetime.
In locations with little light pollution, the moonlit night sky is also blue, because moonlight is reflected sunlight, with a slightly lower color temperature due to the brownish color of the moon. The moonlit sky is not perceived as blue, however, because at low light levels human vision comes mainly from rod cells that do not produce any color perception (Purkinje effect).[citation needed]
[edit]In optical fibers
Rayleigh scattering is an important component of the scattering of optical signals in optical fibers. Silica fibers are disordered materials, thus their density varies on a microscopic scale. The density fluctuations give rise to energy loss due to the scattered light, with the following coefficient:[6]
where n is the refraction index, p is the photoelastic coefficient of the glass, k is the Boltzmann constant, and β is the isothermal compressibility. Tf is afictive temperature, representing the temperature at which the density fluctuations are "frozen" in the material.
[edit]In porous materials
λ−4 Rayleigh-type scattering can also be exhibited by porous materials. An example is the strong optical scattering by nanoporous materials.[8] The strong contrast in refractive index between pores and solid parts of sintered alumina results in very strong scattering, with light completely changing direction each 5 micrometers on average. The λ−4-type scattering is caused by the nanoporous structure (a narrow pore size distribution around ~70 nm) obtained by sintering monodispersive alumina powder.
[edit]See also
- Rayleigh Sky Model
- Rayleigh fading
- Ricean fading
- Raman scattering
- Optical phenomenon
- Dynamic light scattering
- Tyndall effect
- Critical opalescence
- Marian Smoluchowski
- Rayleigh Criterion
- Aerial perspective
http://en.wikipedia.org/wiki/Alumina
Aluminium oxide
From Wikipedia, the free encyclopedia
(Redirected from Alumina)
Aluminium oxide is a chemical compound of aluminium and oxygen with the chemical formula Al2O3. It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium(III) oxide. It is commonly called alumina, and may also be called aloxide, aloxite, or alundum depending on particular forms or applications. It commonly occurs in its crystalline polymorphic phase α-Al2O3, in which it comprises the mineral corundum, varieties of which form the precious gems ruby and sapphire. Al2O3 is significant in its use to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point.[5]
Natural occurrence
Corundum is the most common naturally occurring crystalline form of aluminium oxide. Rubies and sapphiresare gem-quality forms of corundum, which owe their characteristic colors to trace impurities. Rubies are given their characteristic deep red color and their laser qualities by traces of chromium. Sapphires come in different colors given by various other impurities, such as iron and titanium.
[edit]Properties
Al2O3 is an electrical insulator but has a relatively high thermal conductivity (30 Wm−1K−1[1]) for a ceramic material. In its most commonly occurring crystalline form, called corundum or α-aluminium oxide, its hardness makes it suitable for use as anabrasive and as a component in cutting tools.[5]
Aluminium oxide is responsible for the resistance of metallic aluminium to weathering. Metallic aluminium is very reactive with atmospheric oxygen, and a thin passivation layer of aluminium oxide (4 nm thickness) forms on any exposed aluminium surface.[6] This layer protects the metal from further oxidation. The thickness and properties of this oxide layer can be enhanced using a process called anodising. A number of alloys, such as aluminium bronzes, exploit this property by including a proportion of aluminium in the alloy to enhance corrosion resistance. The aluminium oxide generated by anodising is typically amorphous, but discharge assisted oxidation processes such as plasma electrolytic oxidation result in a significant proportion of crystalline aluminium oxide in the coating, enhancing its hardness.
Aluminium oxide is completely insoluble in water. However it is an amphoteric substance, meaning it can react with both acids and bases, such as hydrochloric acid and sodium hydroxide.
- Al2O3 + 6 HCl → 2 AlCl3 + 3 H2O
- Al2O3 + 6 NaOH + 3 H2O → 2 Na3Al(OH)6
Aluminium oxide was taken off the United States Environmental Protection Agency's chemicals lists in 1988. Aluminium oxide is on EPA's Toxics Release Inventory list if it is a fibrous form.[7]
[edit]Structure
[edit]Applications
The great majority of aluminium oxide is consumed for the production of aluminium, usually by the Hall process.
[edit]Filler
Being fairly chemically inert and white, aluminium oxide is a favored filler for plastics. Aluminium oxide is a common ingredient in sunscreen and is sometimes present in cosmetics such as blush, lipstick, and nail polish.
[edit]Catalysis
Aluminium oxide catalyses a variety of reactions that are useful industrially. In its largest scale application, aluminium oxide is the catalyst in the Claus process for converting hydrogen sulfide waste gases into elemental sulfur in refineries. It is also useful for dehydration of alcohols to alkenes.
Aluminium oxide serves as a catalyst support for many industrial catalysts, such as those used in hydrodesulfurization and some Ziegler-Nattapolymerizations.
[edit]Purification
Aluminium oxide is widely used to remove water from gas streams. Other major applications are described below.[17]
[edit]Abrasive
Aluminium oxide is used for its hardness and strength. It is widely used as an abrasive, including as a much less expensive substitute for industrial diamond. Many types of sandpaper use aluminium oxide crystals. In addition, its low heat retention and low specific heat make it widely used in grinding operations, particularly cutoff tools. As the powdery abrasive mineral aloxite, it is a major component, along with silica, of the cue tip "chalk" used in billiards. Aluminium oxide powder is used in some CD/DVD polishing and scratch-repair kits. Its polishing qualities are also behind its use in toothpaste. Aluminium oxide can be grown as a coating on aluminium by anodising or by plasma electrolytic oxidation (see the "Properties" above). Both its strength and abrasive characteristics originate from the high hardness (9 on the Mohs scale of mineral hardness) of aluminium oxide.
[edit]Composite fiber
Aluminium oxide has been used in a few experimental and commercial fiber materials for high-performance applications (e.g., Fiber FP, Nextel 610, Nextel 720).[18] Alumina nanofibers in particular have become a research field of interest.
[edit]See also
http://en.wikipedia.org/wiki/Charged_Aerosol_Release_Experiment
Charged Aerosol Release Experiment
From Wikipedia, the free encyclopedia
The Charged Aerosol Release Experiment also known as CARE, is a project run by NASA which will use a rocket to release of dust in the upper atmosphere to form a dusty plasma in space.[1] NASA plans to trigger cloud formation around the rocket's exhaust particles. [2] The clouds thus generated are intended to simulate naturally occurring phenomena called noctilucent clouds, which are the highest clouds in the atmosphere. The CARE experiment is intended to create an artificial dust layer at the boundary of space in a controlled sense, in order to "allow scientists to study different aspects of it, the turbulence generated on the inside, the distribution of dust particles and such."[3]
The dust cloud is generated using the Nihka motor dust generator. The dust cloud is composed of aluminum oxide, carbon monoxide, hydrogen chloride,water, and nitrogen, as well as smaller amounts of carbon dioxide, hydrogen, monatomic chlorine, and monatomic hydrogen.[4]
According to NASA, SHIMMER will track the CARE dust cloud for days or even months. The SHIMMER instrument has previously viewed natural noctilucent clouds for the past two years. The CARE will be the first space viewing of an artificial noctilucent cloud.[5]
The rocket was set to launch between 7:30 and 7:57 EDT on Tuesday Sept. 14, 2009 from NASA's Wallops Flight Facility in Virginia.
[edit]See also
External links
http://www.youtube.com/watch?v=sQOlSlkgSkk
...this is brendasue signing off from Rainbow Creek. See You Next Time. Big Hugs and Kisses to Everyone. Remember the Motto: Do Not Be Alarmed, Be Informed!!!!!!!!!!!!!!!!!!!!! Love Ya!
Of Course, one more:
http://www.youtube.com/watch?v=hAAq7x06r8k
Sunset of March 7 2013_0001
O+O


























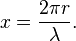



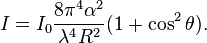






No comments:
Post a Comment
Hi Everybody! Please say hello and follow so I know you are here! Due to the inconsideration of people trying to put commercials on my blog comment area, I have restricted use of anonymous posts. Sorry that some hurt all.
My public email is katescabin@gmail.com No spammers or trolls