
SNAP, PRESTO:
Look Where We Are!!! Have a look around! Just Push Play to Begin Our Day
Welcome to Scotland. Our Host:
David wilson – Glasgow Botanic Gardens Vol 5
https://plus.google.com Glasgow Botanic Gardens Vol 5 Botanic Gardens Glasgow Vol. 5 (12 photos)David wilson – Jul 24, 2012 –
Glasgow Botanic Gardens Vol 5 Botanic Gardens Glasgow Vol. 5 (12 photos)David wilson – Jul 24, 2012 –
Gardenvisit Editorial
Located in the city's West End, Glasgow Botanic is a cross between a public park and a botanic garden, adorned by a great conservatory: the Kibble Palace of 1873. Kibble Palace underwent extensive restoration between 2003 and 2006. It houses an important national collection of Australasian tree ferns as well as other exotics. The garden was established in 1817 to supply resources to the University of Glasgow. William Hooker held the post of regius professor at Glasgow Botanic Gardens before his appointment as the first director of Kew Gardens.
Address - 730 Great Western Road, Glasgow, Strathclyde, Scotland, G12 0UE
Opening times - All year, Daily, Open 7am to dusk
Admission - Entrance free
Website - Visit the Glasgow Botanic Gardens website
Opening times - All year, Daily, Open 7am to dusk
Admission - Entrance free
Website - Visit the Glasgow Botanic Gardens website
Check It Out!!!!!!
http://en.wikipedia.org/wiki/Glasgow_Botanic_Gardens

Glasgow Botanic Gardens
From Wikipedia, the free encyclopedia
Glasgow Botanic Gardens is an Arboretum and public park located in the West End of Glasgow, Scotland. It features several glasshouses, the most notable of which is the Kibble Palace. The gardens were created in 1817, and run by the Royal Botanic Institution of Glasgow (founded by Thomas Hopkirk of Dalbeth), and were intended to supply the University of Glasgow.William Hooker was regius professor of botany at Glasgow University, and contributed to the development of the Botanic Gardens before his appointment to the directorship of Kew Gardens in London.[1] The gardens were originally used for concerts and other events, and in 1891 the gardens were incorporated in to the Parks and Gardens of the City of Glasgow.
The site was once served by a railway line, and Botanic Gardens Railway Station remains today in a derelict state as a remarkable example of a disused station. It is hidden behind some trees and a metal fence blocks access to the platforms. Kirklee railway station also lies just inside the gardens.
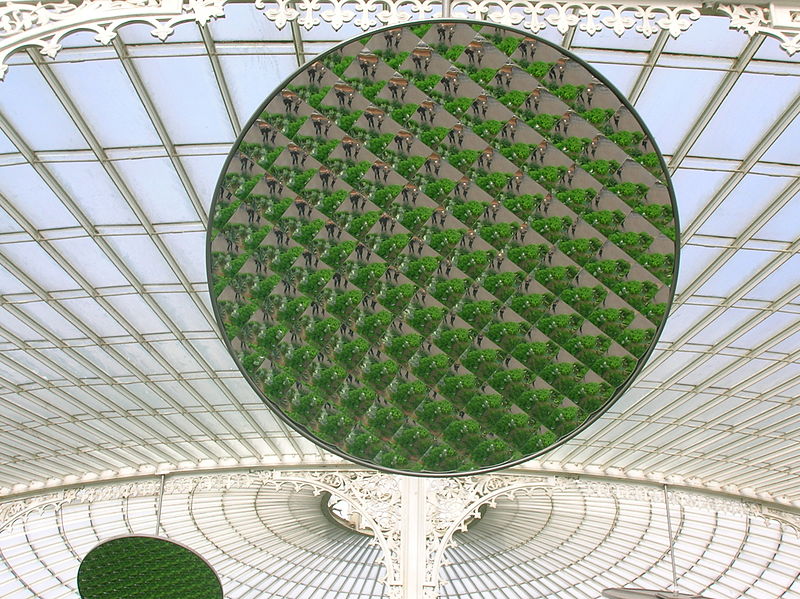
A reflective multi-facet mirror in the restored Kibble Palace.
Kibble Palace
Kibble Palace is a 19th century wrought iron framed glasshouse, covering 2137 m.2 Originally designed by John Kibble for his home atCoulport on Loch Longin the 1860s,[2] the components were cast by Walter Macfarlaneat his Saracen Foundry in Possilpark. Eventually brought up the River Clyde by barge to the Botanic Gardens, it was fully erected at its current location in 1873 by Boyd of Paisley.[3]
The building structure is of curved wrought iron and glass supported by cast iron beams resting on ornate columns, surmounted on masonry foundations. It was initially used as an exhibition and concert venue, before being used for growing plants from the 1880s.Benjamin Disraeli and William Ewart Gladstone were both installed as rectors of the University of Glasgow in the palace, in 1873 and 1879 respectively - its last use as a public events venue, before becoming wholly used for the cultivation of temperate plants. The main plant group is the collection of Australian tree ferns, some of which have lived here for 120 years.
In the 1920s a statue was added in the palace to "King Robert of Sicily" a figure from the works of Longfellow. This is by the Scottish sculptor George Henry Paulin.
In 2004 a £7 million restoration programme was initiated to repair corrosion of the ironwork. The restoration involved the complete dismantling of the Palace, and the removal of the parts to Shafton, South Yorkshire for specialised repair and conservation. The plant collection was removed completely for the first time ever and the ironwork was rebuilt over a rearranged floorplan, giving the Palace a prolonged life. It re-opened to the public in November 2006.

Main range of glasshouses
Just Push Play
Just Push Play
David Wilson. Here is the Link to Profile:
The Botanic Gardens in Glasgow with
You and Mrs Wilson! I love the Nature
Trips the two of You get to see!
David has 10 albums in his G+ Gallery from
this wonderful place, so be sure to stop
by and see them all.
Tonight, he has shared his photostudy of
their adventure! You are in for a treat of
some of the finest photos ever of these
flowers. Enjoy the trip!

Lived in Glasgow

https://plus.google.com/109221698061777003785/posts
This is one reason I adore David and Mrs Wilson!
This is one reason I adore David and Mrs Wilson!









David Captures Killer Plants!!!!!!!!!!!!








True carnivory is thought to have evolved independently six times in five different orders of flowering plants,[2][3] and these are now represented by more than a dozen genera. These include about 630 species that attract and trap prey, produce digestive enzymes, and absorb the resulting available nutrients.[4] Additionally, over 300 protocarnivorous plant species in several genera show some but not all these characteristics.Carnivorous plants are plants that derive some or most of their nutrients (but not energy) from trapping and consuming animals or protozoans, typically insects and other arthropods. Carnivorous plants have adapted to grow in places where the soil is thin or poor in nutrients, especially nitrogen, such as acidic bogs and rock outcroppings. Charles Darwin wroteInsectivorous Plants, the first well-known treatise on carnivorous plants, in 1875.[1]
Carnivorous plant
From Wikipedia, the free encyclopedia
Trapping mechanisms
Five basic trapping mechanisms are found in carnivorous plants.
- Pitfall traps (pitcher plants) trap prey in a rolled leaf that contains a pool of digestive enzymes or bacteria.
- Flypaper traps use a sticky mucilage.
- Snap traps utilize rapid leaf movements.
- Bladder traps suck in prey with a bladder that generates an internal vacuum.
- Lobster-pot traps force prey to move towards a digestive organ with inward-pointinghairs.
These traps may be active or passive, depending on whether movement aids the capture of prey. For example, Triphyophyllum is a passive flypaper that secretes mucilage, but whose leaves do not grow or move in response to prey capture. Meanwhile, sundews are active flypaper traps whose leaves undergo rapid acid growth, which is an expansion of individual cells as opposed to cell division. The rapid acid growth allows the sundew tentacles to bend, aiding in the retention and digestion of prey.[5]
[edit]Pitfall traps
Main article: Pitcher plant
Pitfall traps are thought to have evolved independently on at least four occasions. The simplest ones are probably those of Heliamphora, the marsh pitcher plant. In this genus, the traps are clearly derived evolutionarily from a simple rolled leaf whose margins have sealed together. These plants live in areas of high rainfall in South America such as Mount Roraima and consequently have a problem ensuring their pitchers do not overflow. To counteract this problem, natural selection has favoured the evolution of an overflow similar to that of a bathroom sink—a small gap in the zipped-up leaf margins allows excess water to flow out of the pitcher.
Heliamphora is a member of the Sarraceniaceae, a New World family in the order Ericales (heathers and allies). Heliamphora is limited to South America, but the family contains two other genera, Sarracenia and Darlingtonia, which are endemic to the Southeastern United States (with the exception of one species) and California respectively. Sarracenia purpurea subsp. purpurea (the northern pitcher plant) can be found as far north as Canada. Sarracenia is the pitcher plant genus most commonly encountered in cultivation, because it is relatively hardy and easy to grow.
In the genus Sarracenia, the problem of pitcher overflow is solved by an operculum, which is essentially a flared leaflet that covers the opening of the rolled-leaf tube and protects it from rain. Possibly because of this improved waterproofing, Sarracenia species secrete enzymes such as proteases and phosphatases into the digestive fluid at the bottom of the pitcher;Heliamphora relies on bacterial digestion alone. The enzymes digest the proteins and nucleic acids in the prey, releasing amino acids and phosphate ions, which the plant absorbs.
Darlingtonia californica, the cobra plant, possesses an adaptation also found in Sarracenia psittacina and, to a lesser extent, in Sarracenia minor: the operculum is balloon-like and almost seals the opening to the tube. This balloon-like chamber is pitted with areolae,chlorophyll-free patches through which light can penetrate. Insects, mostly ants, enter the chamber via the opening underneath the balloon. Once inside, they tire themselves trying to escape from these false exits, until they eventually fall into the tube. Prey access is increased by the "fish tails", outgrowths of the operculum that give the plant its name. Some seedling Sarracenia species also have long, overhanging opercular outgrowths; Darlingtoniamay therefore represent an example of neoteny.
The second major group of pitcher plants are the monkey cups or tropical pitcher plants of the genus Nepenthes. In the hundred or so species of this genus, the pitcher is borne at the end of a tendril, which grows as an extension to the midrib of the leaf. Most species catch insects, although the larger ones, such as Nepenthes rajah, also occasionally take smallmammals and reptiles. Nepenthes bicalcarata possesses two sharp thorns that project from the base of the operculum over the entrance to the pitcher. These likely serve to lure insects into a precarious position over the pitcher mouth, where they may lose their footing and fall into the fluid within.[6]
The pitfall trap has evolved independently in at least two other groups. The Albany pitcher plant Cephalotus follicularis is a small pitcher plant from Western Australia, with moccasin-like pitchers. The rim of its pitcher's opening (the peristome) is particularly pronounced (both secrete nectar) and provides a thorny overhang to the opening, preventing trapped insects from climbing out. The lining of most pitcher plants is covered in a loose coating of waxyflakes, which are slippery for insects, prey that are often attracted by nectar bribes secreted by the peristome and by bright flower-like anthocyanin patterning. In at least one species,Sarracenia flava, the nectar bribe is laced with coniine, a toxic alkaloid also found inhemlock, which probably increases the efficiency of the traps by intoxicating prey.[7]
The final carnivore with a pitfall-like trap is the bromeliad Brocchinia reducta. Like most relatives of the pineapple, the tightly-packed, waxy leaf bases of the strap-like leaves of this species form an urn. In most bromeliads, water collects readily in this urn and may provide habitats for frogs, insects and, more useful for the plant, diazotrophic (nitrogen-fixing) bacteria. In Brocchinia, the urn is a specialised insect trap, with a loose, waxy lining and a population of digestive bacteria.[citation needed]
[edit]Flypaper traps
The flypaper trap is based on a sticky mucilage, or glue. The leaf of flypaper traps is studded withmucilage-secreting glands, which may be short and nondescript (like those of the butterworts), or long and mobile (like those of many sundews). Flypapers have evolved independently at least five times.
In the genus Pinguicula, the mucilage glands are quite short (sessile), and the leaf, while shiny (giving the genus its common name of 'butterwort'), does not appear carnivorous. However, this belies the fact that the leaf is an extremely effective trap of small flying insects (such as fungus gnats), and its surface responds to prey by relatively rapid growth. This thigmotropic growth may involve rolling of the leaf blade (to prevent rain from splashing the prey off the leaf surface) or dishing of the surface under the prey to form a shallow digestive pit.
The sundew genus (Drosera) consists of over 100 species of active flypapers whose mucilage glands are borne at the end of long tentacles, which frequently grow fast enough in response to prey (thigmotropism) to aid the trapping process. The tentacles of D. burmanii can bend 180° in a minute or so. Sundews are extremely cosmopolitan and are found on all the continents except the Antarctic mainland. They are most diverse in Australia, the home to the large subgroup of pygmy sundews such as D. pygmaea and to a number of tuberous sundews such as D. peltata, which form tubers that aestivate during the dry summer months. These species are so dependent on insect sources of nitrogen that they generally lack the enzyme nitrate reductase, which most plants require to assimilate soil-borne nitrate into organic forms.
Closely related to Drosera is the Portuguese dewy pine, Drosophyllum, which differs from the sundews in being passive. Its leaves are incapable of rapid movement or growth. Unrelated, but similar in habit, are the Australian rainbow plants (Byblis). Drosophyllum is unusual in that it grows under near-desert conditions; almost all other carnivores are either bog plants or grow in moist tropical areas.
Recent molecular data (particularly the production of plumbagin) indicate that the remainingflypaper, Triphyophyllum peltatum, a member of the Dioncophyllaceae, is closely related toDrosophyllum and forms part of a larger clade of carnivorous and non-carnivorous plants with the Droseraceae, Nepenthaceae, Ancistrocladaceae and Plumbaginaceae. This plant is usually encountered as a liana, but in its juvenile phase, the plant is carnivorous. This may be related to a requirement for specific nutrients for flowering.
[edit]Snap traps
The only two active snap traps—the Venus flytrap (Dionaea muscipula) and the waterwheel plant (Aldrovanda vesiculosa)—are believed to have had a common ancestor with similar adaptations. Their trapping mechanism has also been described as a "mouse trap", "bear trap" or "man trap", based on their shape and rapid movement. However, the term snap trapis preferred as other designations are misleading, particularly with respect to the intended prey. Aldrovanda is aquatic and specialised in catching small invertebrates; Dionaea is terrestrial and catches a variety of arthropods, including spiders.[8]
The traps are very similar, with leaves whose terminal section is divided into two lobes, hinged along the midrib. Trigger hairs (three on each lobe in Dionaea muscipula, many more in the case of Aldrovanda) inside the trap lobes are sensitive to touch. When a trigger hair is bent, stretch-gated ion channels in the membranes of cells at the base of the trigger hair open, generating an action potential that propagates to cells in the midrib.[9] These cells respond by pumping out ions, which may either cause water to follow by osmosis (collapsing the cells in the midrib) or cause rapid acid growth.[10] The mechanism is still debated, but in any case, changes in the shape of cells in the midrib allow the lobes, held under tension, to snap shut,[9] flipping rapidly from convex to concave[11] and interring the prey. This whole process takes less than a second. In the Venus flytrap, closure in response to raindrops and blown-in debris is prevented by the leaves having a simple memory: for the lobes to shut, two stimuli are required, 0.5 to 30 seconds apart.[citation needed]
The snapping of the leaves is a case of thigmonasty (undirected movement in response to touch). Further stimulation of the lobe's internal surfaces by the struggling insects causes the lobes to close even tighter (thigmotropism), sealing the lobes hermetically and forming a stomach in which digestion occurs over a period of one to two weeks. Leaves can be reused three or four times before they become unresponsive to stimulation, depending on the growing conditions.
[edit]Bladder traps
Bladder traps are exclusive to the genus Utricularia, or bladderworts. The bladders (vesicula) pump ions out of their interiors. Water follows by osmosis, generating a partial vacuum inside the bladder. The bladder has a small opening, sealed by a hinged door. In aquatic species, the door has a pair of long trigger hairs. Aquatic invertebrates such as Daphnia touch these hairs and deform the door by lever action, releasing the vacuum. The invertebrate is sucked into the bladder, where it is digested. Many species of Utricularia (such as U. sandersonii) are terrestrial, growing on waterlogged soil, and their trapping mechanism is triggered in a slightly different manner. Bladderworts lack roots, but terrestrial species have anchoring stems that resemble roots. Temperate aquatic bladderworts generally die back to a restingturion during the winter months, and U. macrorhiza appears to regulate the number of bladders it bears in response to the prevailing nutrient content of its habitat.
[edit]Lobster-pot traps
A lobster-pot trap is a chamber that is easy to enter, and whose exit is either difficult to find or obstructed by inward-pointing bristles. Lobster pots are the trapping mechanism inGenlisea, the corkscrew plants. These plants appear to specialise in aquatic protozoa. A Y-shaped modified leaf allows prey to enter but not exit. Inward-pointing hairs force the prey to move in a particular direction. Prey entering the spiral entrance that coils around the upper two arms of the Y are forced to move inexorably towards a stomach in the lower arm of the Y, where they are digested. Prey movement is also thought to be encouraged by water movement through the trap, produced in a similar way to the vacuum in bladder traps, and probably evolutionarily related to it.
Outside of Genlisea, features reminiscent of lobster-pot traps can be seen in Sarracenia psittacina, Darlingtonia californica, and, some horticulturalists argue, Nepenthes aristolochioides.
[edit]Borderline carnivores
Main article: Protocarnivorous plant
To be a fully fledged carnivore, a plant must attract, kill, and digest prey;[2][12] and it must benefit from absorbing the products of the digestion (mostly amino acids and ammonium ions).[13] To many horticulturalists, these distinctions are a matter of taste. There is a spectrum of carnivory found in plants: from completely non-carnivorous plants like cabbages, to borderline carnivores, to unspecialised and simple traps, like Heliamphora, to extremely specialised and complex traps, like that of the Venus flytrap.
The borderline carnivores include Roridula and Catopsis berteroniana. Catopsis is a borderline carnivorous bromeliad, like Brocchinia reducta. However, unlike B. reducta, which produces the enzyme phosphatase, C. berteroniana has not been shown to produce any digestive enzymes at all.[14] In these pitfall traps, prey simply fall into the urn, assisted by the waxy scales located on the rim. Roridula has a more intricate relationship with its prey. The plants in this genus produce sticky leaves with resin-tipped glands and look extremely similar to some of the larger sundews. However, they do not directly benefit from the insects they catch. Instead, they form amutualistic symbiosis with species of assassin bug (genus Pameridea), which eat the trapped insects. The plant benefits from the nutrients in the bugs' faeces.[15]
A number of species in the Martyniaceae (previously Pedaliaceae), such as Ibicella lutea, have sticky leaves that trap insects. However, these plants have not been shown conclusively to be carnivorous.[16] Likewise, the seeds of Shepherd's Purse,[16] urns of Paepalanthus bromelioides,[17] bracts of Passiflora foetida,[18] and flower stalks and sepals of triggerplants(Stylidium)[19] appear to trap and kill insects, but their classification as carnivores is contentious.
The production of specific prey-digesting enzymes (proteases, ribonucleases, phosphatases, etc.) is sometimes used as a criterion for carnivory. However, this would probably discount Heliamphora[20] and Darlingtonia,[21] both of which appear to rely on the enzymes ofsymbiotic bacteria to break down their prey but are generally considered as carnivores. However, discounting the enzyme-based definition leaves open the question of Roridula.
[edit]Evolution
The evolution of carnivorous plants is obscured by the paucity of their fossil record. Very fewfossils have been found, and then usually only as seed or pollen. Carnivorous plants are generally herbs, and their traps primary growth. They generally do not form readily fossilisable structures such as thick bark or wood. The traps themselves would probably not be preserved in any case.
Still, much can be deduced from the structure of current traps. Pitfall traps are quite clearly derived from rolled leaves. The vascular tissues of Sarracenia is a case in point. The keel along the front of the trap contains a mixture of leftward- and rightward-facing vascular bundles, as would be predicted from the fusion of the edges of an adaxial (stem-facing) leaf surface. Flypapers also show a simple evolutionary gradient from sticky, non-carnivorous leaves, through passive flypapers to active forms. Molecular data show theDionaea–Aldrovanda clade is closely related to Drosera,[22] but the traps are so dissimilar that the theory of their origin—very fast-moving flypapers became less reliant on glue—remains rather speculative.
There are over a quarter of a million species of flowering plants. Of these, only around 630 are known to be carnivorous. True carnivory has probably evolved independently at least six times;[2] however, some of these "independent" groups probably descended from a recent common ancestor with a predisposition to carnivory. Some groups (the Ericales andCaryophyllales) seem particularly fertile ground for carnivorous preadaptation, although in the former case, this may be more to do with the ecology of the group than its morphology, as most of the members of this group grow in low-nutrient habitats such as heath and bog.
It has been suggested that all trap types are modifications of a similar basic structure—the hairy leaf.[23] Hairy (or more specifically, stalked-glandular) leaves can catch and retain drops of rainwater, especially if shield-shaped or peltate, thus promoting bacteria growth. Insects land on the leaf, become mired by the surface tension of the water, and suffocate. Bacteria jumpstart decay, releasing from thecorpse nutrients that the plant can absorb through its leaves. This foliar feeding can be observed in most non-carnivorous plants. Plants that were better at retaining insects or water therefore had a selective advantage. Rainwater can be retained by cupping the leaf, leading to pitfall traps. Alternatively, insects can be retained by making the leaf stickier by the production of mucilage, leading to flypaper traps.
The pitfall traps may have evolved simply by selection pressure for the production of more deeply cupped leaves, followed by "zipping up" of the margins and subsequent loss of most of the hairs, except at the bottom, where they help retain prey.
The lobster-pot traps of Genlisea are difficult to interpret. They may have developed from bifurcated pitchers that later specialised on ground-dwelling prey; or, perhaps, the prey-guiding protrusions of bladder traps became more substantial than the net-like funnel found in most aquatic bladderworts. Whatever their origin, the helical shape of the lobster pot is an adaptation that displays as much trapping surface as possible in all directions when buried in moss.
The traps of the bladderworts may have derived from pitchers that specialised in aquatic prey when flooded, like Sarracenia psittacina does today. Escaping prey in terrestrial pitchers have to climb or fly out of a trap, and both of these can be prevented by wax, gravity and narrow tubes. However, a flooded trap can be swum out of, so in Utricularia, a one-way lid may have developed to form the door of a proto-bladder. Later, this may have become active by the evolution of a partial vacuum inside the bladder, tripped by prey brushing against trigger hairs on the door of the bladder.
Flypaper traps include the various true flypapers and the snap traps of Aldrovanda andDionaea. The production of sticky mucilage is found in many non-carnivorous genera, and the passive glue traps in Byblis and Drosophyllum could easily have evolved.
The active glue traps use rapid plant movements to trap their prey. Rapid plant movement can result from actual growth, or from rapid changes in cell turgor, which allow cells to expand or contract by quickly altering their water content. Slow-moving flypapers like Pinguicula exploit growth, but the Venus flytrap uses such rapid turgor changes that glue became unnecessary. The stalked glands that once made it and which are so evident in Drosera have become the teeth and trigger hairs—an example of natural selection hijacking preexisting structures for new functions.
Recent taxonomic analysis[24] of the relationships within the Caryophyllales indicate that theDroseraceae, Triphyophyllum, Nepenthaceae and Drosophyllum, while closely related, are embedded within a larger clade that includes non-carnivorous groups such as the tamarisks,Ancistrocladaceae, Polygonaceae and Plumbaginaceae. Interestingly, the tamarisks possess specialised salt-excreting glands on their leaves, as do several of the Plumbaginaceae (such as the sea lavender, Limonium), which may have been co-opted for the excretion of other chemicals, such as proteases and mucilage. Some of the Plumbaginaceae (e.g. Ceratostigma) also have stalked, vascularised glands that secrete mucilage on their calyces and aid in seed dispersal and possibly in protecting the flowers from crawling parasitic insects. These are probably homologous with the tentacles of the carnivorous genera. Perhaps carnivory evolved from a protective function, rather than a nutritional one. The balsams (such as Impatiens), which are closely related to the Sarraceniaceae and Roridula, similarly possess stalked glands.
The only traps that are unlikely to have descended from a hairy leaf or sepal are the carnivorous bromeliads (Brocchinia and Catopsis). These plants use the urn—a fundamental part of a bromeliad—for a new purpose and build on it by the production of wax and the other paraphernalia of carnivory.
[edit]
FEATURE PRESENTATION!!!!!!!!!!!
This is sooooooo fantastic! Just Push Play
Another great vid from England-Just Push Play
These plants are sooooo cool!!!!!! Just Push Play
Okay, we have to go now!
Off to the Airport in Glasgow!
Bye David and Mrs Wilson!
Thanks for a great Trip! Great Photos!
Just Push Play for Departure!
....this is brendasue signing off from Rainbow
Creek! See You next time! Love to All
Of course one, two, three more Performances!!
Remember do not buy any Man Eating
Plants!!!!!!!!! Just Push Play!
SMILE MORE!
This is sooooooo fantastic! Just Push Play
Off to the Airport in Glasgow!
Bye David and Mrs Wilson!
Thanks for a great Trip! Great Photos!
Just Push Play for Departure!
Creek! See You next time! Love to All
Of course one, two, three more Performances!!
Remember do not buy any Man Eating
Plants!!!!!!!!! Just Push Play!
Just Push Play
Just Push Play
O+O
















No comments:
Post a Comment
Hi Everybody! Please say hello and follow so I know you are here! Due to the inconsideration of people trying to put commercials on my blog comment area, I have restricted use of anonymous posts. Sorry that some hurt all.
My public email is katescabin@gmail.com No spammers or trolls